High quality manufacturing standards will help skyrocket your e-liquid brand. Customers expect top-of-the-range products. These are the only ones they are willing to buy, are loyal to and want to explore new ones. We have been in business since 2013 and our partners get products of repeatable quality. Get some tips from wordclass-level e-liquid manufacturer (and more!).
Learn more:
- OEM Services And Private & White Label: 9 Reasons To Choose Chemnovatic As The Best Business Partner
- How To Start And Grow E-Liquid Brand?
- Pharmaceutical Quality Of E-Liquids: What You Should Know?
- Packaging, Storing And Transport: Why Are They Important For Product’s Quality?
- Storing And Handling Pure Nicotine And Nicotine Salts
- Safe Usage Of Chemicals [Guide]
- E-Liquid Flavors To Make Your Recipes Stand Out
- Natural Vaping Products: Bases, Nicotine, Flavorings
- PG/VG Ratio: How To Find The Best For E-Liquids
- The Differences Between Glycol And Glycerine: When To Use Which?
- E-Liquid Regulations In The World
E-Liquid Manufacturer: Quality Management System Is Crucial
First of all, it is important to implement a quality management system for delivering stable quality. It is a system of rules, procedures, methods, tools, job descriptions, people, and relations between them. This is to achieve the set quality goals. And the main document in the quality management system is the quality policy, which is one of the documents of the Quality Manual.
Chemnovatic quality policy
To give a good example, let me quote the Chemnovatic quality policy. For instance, in the introduction, we can read:
“The aim of Chemnovatic Sp. z o.o. Sp. k.is to constantly strive to satisfy our clients with the services and products we offer, i.e. the production and distribution of liquids for electronic cigarettes and raw materials for their production. We make sure that our services and products meet the current and future expectations of our customers, and in particular their safety expectations. For this purpose, we have implemented GMP, GHP HACCP quality assurance systems, and a quality management system compliant with the ISO 9001: 2015 standard.”
See also:
- Industry Regulations: REACH And CLP
- Fees, Taxes And More: An Analysis Of Selected Regulations In Europe
- Tobacco Product Directive (TPD): All You Need To Know
- What Are E-Liquids? History And Business Opportunities
- What Is Nicotine And How Is It Classified?
- From Leaves To Labs: A Brief Story About Nicotine’s Production
- Hard To Handle, Easy To Vape: How To Work With Nicotine Safely?
- What Is Nicotine Base And How To Use It
- 5 Steps To High Quality Nicotine Bases
Is certified production necessary to improve e-liquid quality standards?
The law does not require the certified production of e-liquids. However, if you want to be a real pro in the e-liquids industry and be able to deliver stable, high-quality products, you should implement a system for maintaining high manufacturing standards.
You can be sure that users will notice any quality irregularities. And this, in turn, may result in losing trust in the brand. Which may have dramatic effects on sales.
How do we achieve top quality standards? We have implemented GMP/GHP and HACCP. To clarify, in November 2017 Chemnovatic has been subjected to the certification audit by TÜV Rheinland Polska Sp. z o. o., as a result, we have been awarded the ISO 9001:2015, GMP, GHP, HACCP certificates.
See also:
Moreover, we are constantly improving the production and management processes to increase the quality of our products. ISO International Standards ensure safety, reliability, and quality of products. To clarify, it is a set of strategic tools minimizing waste and human errors while increasing productivity. On the other hand, the HACCP system is a method of conduct and a tool ensuring product safety and hygiene.
Coupled with the implementation of the principles of Good Hygienic Practice and Good Manufacturing Practice (GHP/GMP), it can be perceived as an integrated system of activities related to the management of product safety.
Learn more:
HACCP – Hazard Analysis and Critical Control Point
It is the system, which identifies, assesses, and controls threats essential for food safety and health quality (Food Code). HACCP is a method of ensuring the safety of food that relies primarily on risk prevention. In other words, it helps to ensure process reliability production, packaging, storage, and distribution of food. The HACCP system is beneficial for both the consumer and the entrepreneur. Moreover, the benefits of implementing the HACCP system, in addition to the overriding goal of ensuring food safety, include:
- meeting the requirements of food law,
- updating knowledge and raising awareness of staff,
- the discipline of the crew and tightening of cooperation between people in particular positions,
- meeting customer expectations, increasing the certainty and systematic repeatability of product quality,
- an active approach to solving quality problems, and product safety,
- enabling remedial actions before the problem occurs,
- improvement of the company’s infrastructure,
- reduction of production losses, errors, and shortages.
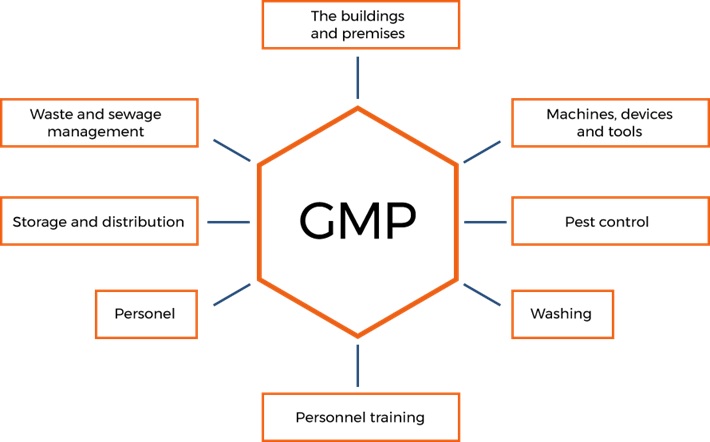
GHP/GMP principles specify actions for improving e-liquid manufacturer quality standards
Good Hygiene Practice and Good Manufacturing Practice. Actions, which must be taken, and hygienic conditions, which must be met and controlled at all production or marketing stages to ensure the safety of food. The principles of Good Manufacturing Practice (GMP) take into account the principles of Good Hygiene Practice (GHP).
What do these certificates mean for customers?
The application of GMP/GHP, and HACCP standards gives consumers a greater level of comfort and certainty as to the quality of the product manufactured in a plant where Good Manufacturing Practices are applied. For example, the consumer can be sure that the product is completely safe.
What’s more, by having a proper and well-functioning system, an entrepreneur builds consumer confidence in the company and improves its image.
Want to know more about standards for e-liquid manufacturer?
If you want to learn more, check out our guide about becoming a brand owner! or visit our store to check the top-quality raw materials for vape industry.
We will help take your business to the next level. We’ll take care of it from A to Z; from recipe, to production, to laboratory testing, packaging and promotion. Simply, take advantage of our OEM services!
Let’s grow your business together!
Subscribe to our newsletter and receive a free access to our e-mail course on raw materials for e-liquids production (and more!).
No spam, only valuable content we promise to send you.