With the new Tobacco Products Directive (TPD) on the way, there is much confusion among the e-liquid manufacturers: “What do I need to do to be TPD compliant?”, “How do I label and package my e-liquids according to TPD?” or “Is my e-liquid packaging good enough for TPD?” and similar questions being raised every day. At CHEMNOVATIC we got the requirements for “TPD ready” e-liquid packaging worked out and can proudly say that our customers’ products are all meeting TPD requirements. The following article is a boiled- down version of TPD guidelines with a touch of our experience – TPD done our way.
Learn more:
- OEM Services And Private & White Label: 9 Reasons To Choose Chemnovatic As The Best Business Partner
- Tobacco Product Directive (TPD): All You Need To Know
- E-Liquid Regulations In The World
- E-Liquid Regulations Guide: [Get Your Free E-Book]
- E-Liquid Industry Regulations: REACH and CLP
- Fees, Taxes And More: An Analysis Of Selected Regulations In Europe
- Excise Duty In The Vape Industry [Guide]
- What Is CLP?
- What Is UFI Code And How Does It Apply To The E-Liquid Industry?
- REACH: What Is Reach And Why We Talk About It?
- E-Liquid Bottles Nozzle Requirements
- Nicotine Shots: Dealing With TPD Regulations
Remember: The following regulations are the general guidelines introduced by the EU. Member states have the right to introduce their own, stricter regulations- please make sure to acquaint yourself with the regulations appropriate for your country.
E-liquid Packaging: Labels
Warnings and pictograms depend on the CLP classification method. First CLP compliant method of nicotine classification: For ALL e-liquids with nicotine strengths up to 20mg/ml: One of the following warnings should appear on the label:
- “If medical advice is needed, have product container or label at hand.”
- “Product contains nicotine”
Second CLP compliant method of nicotine classification: For e-liquids with strengths of 3mg/ml:
- a pictogram: with WARNING written below

- “Product contains nicotine”
- “Harmful in contact with skin. Wear protective gloves/ protective clothing. IF SWALLOWED: Immediately call a POISON CENTER or doctor/ physician. IF ON SKIN: Wash with plenty of soap and water. Wash contaminated clothing before reuse. Dispose of contents/ container to properly labeled waste containers in accordance with national legislation.”
For e-liquids with strengths of 6mg/ml:
- a pictogram: with DANGER written below

- “Product contains nicotine”
- “Toxic in contact with skin. Wear protective gloves/ protective clothing. IF SWALLOWED: Immediately call POISON CENTER or doctor/ physician. IF ON SKIN: Wash with plenty of soap and water. Wash contaminated clothing before reuse. Dispose of contents/ container to properly labeled waste containers in accordance with national legislation.”
We have been on the market since 2013. We know the vape and e-liquid industry inside out. That is why we have created a platform where we share all the knowledge and experience we have gained in a professional and understandable way – step by step. Check out our Knowledge Base now!
Example of our label within minimum required information
- VAPY
- Ingredients: propylene glycol, vegetable glycerin, flavorings, nicotine
- Nicotine content: Depending on e-liquid strength, specified in mg/ml
- Nicotine content in ingested dose: Depending on e-liquid strength, specified in percent (%)
- Batch number: 201511240000, Best before: 23.11.2017
- Product not to be sold to people under the age of 18. Keep out of reach of children and animals.
- CHEMNOVATIC Sp. z o. o. Sp. k. Ludwika Spiessa 9, 20-270 Lublin www.chemnovatic.com Tel. +48 81 475 44 42
- Product contains nicotine which is a highly addictive substance. Not recommended for non- smokers.
Individual packaging for e-liquids
Individual packaging must contain the following:
- Brand name
- Ingredients listed in descending order by weight
- Nicotine content
- Nicotine content in ingested dose
- Batch number
- “Keep out of reach of children and animals.”
- Manufacturers information: name, address, phone number, www address.
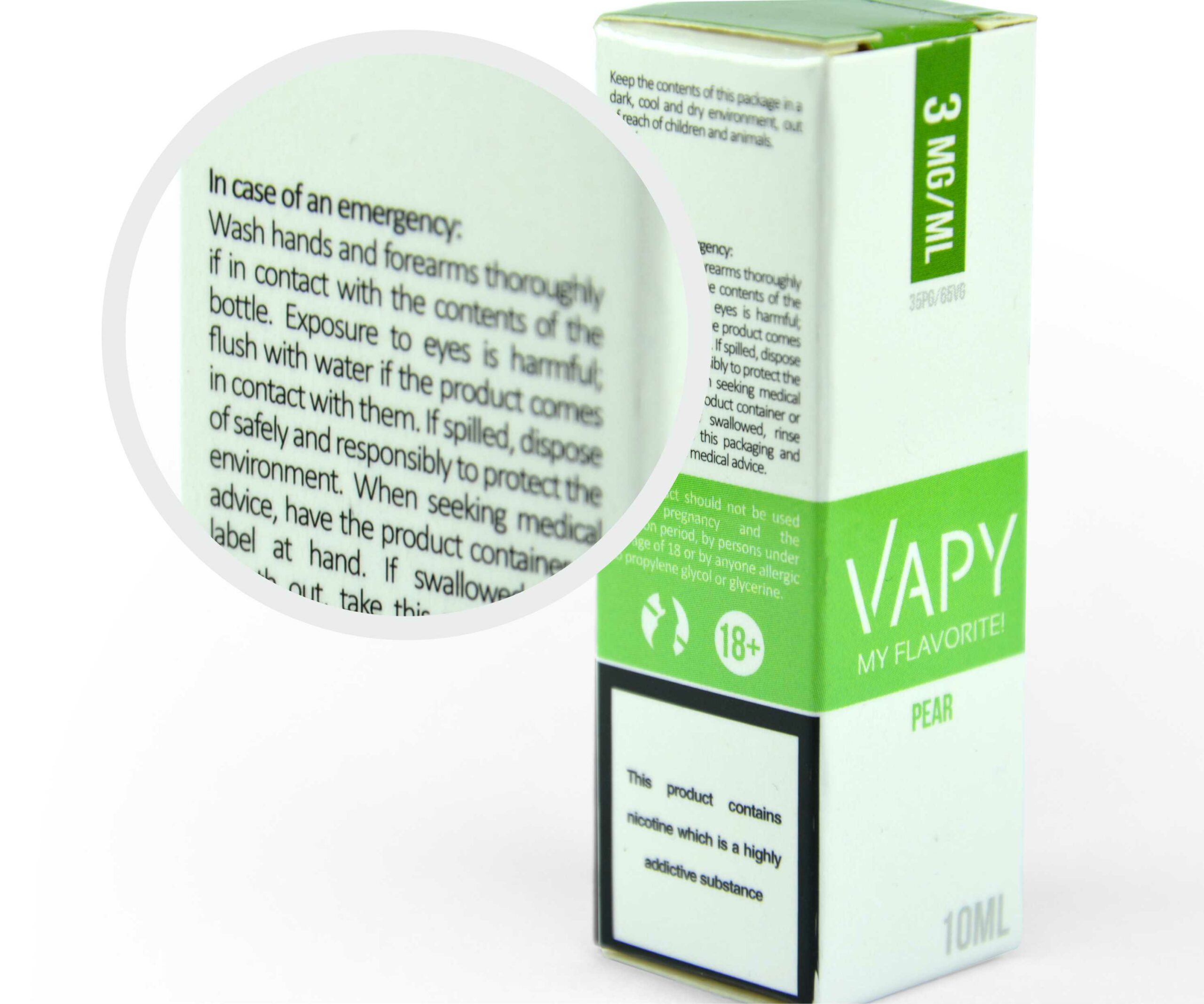 Health warning: “This product contains nicotine which is a highly addictive substance, it is not recommended to be sold to non- smokers.” (written in black, bold Helvetica font on white background; centered within the designated area, for cubical and collective packaging the warnings are parallel to the side edges of the individual or collective packaging. The warning text is parallel to the main text on the plane reserved for that warning. Health warning: visible on two biggest planes of the individual and collective packaging, takes up 30% of individual and collective packaging area.)
Health warning: “This product contains nicotine which is a highly addictive substance, it is not recommended to be sold to non- smokers.” (written in black, bold Helvetica font on white background; centered within the designated area, for cubical and collective packaging the warnings are parallel to the side edges of the individual or collective packaging. The warning text is parallel to the main text on the plane reserved for that warning. Health warning: visible on two biggest planes of the individual and collective packaging, takes up 30% of individual and collective packaging area.)
Additional content to be displayed on individual e-liquid packaging
- 35%PG/ 65%VG
- “Detailed information can be found on the attached flyer.” (label version)
- “Detailed information can be found on a flyer inside the box.” (individual packaging version)
- “18+” pictogram

- “Not to be used during pregnancy” pictogram

- “Recycling” pictogram
- Individual packaging warnings: “E-liquid is a substance
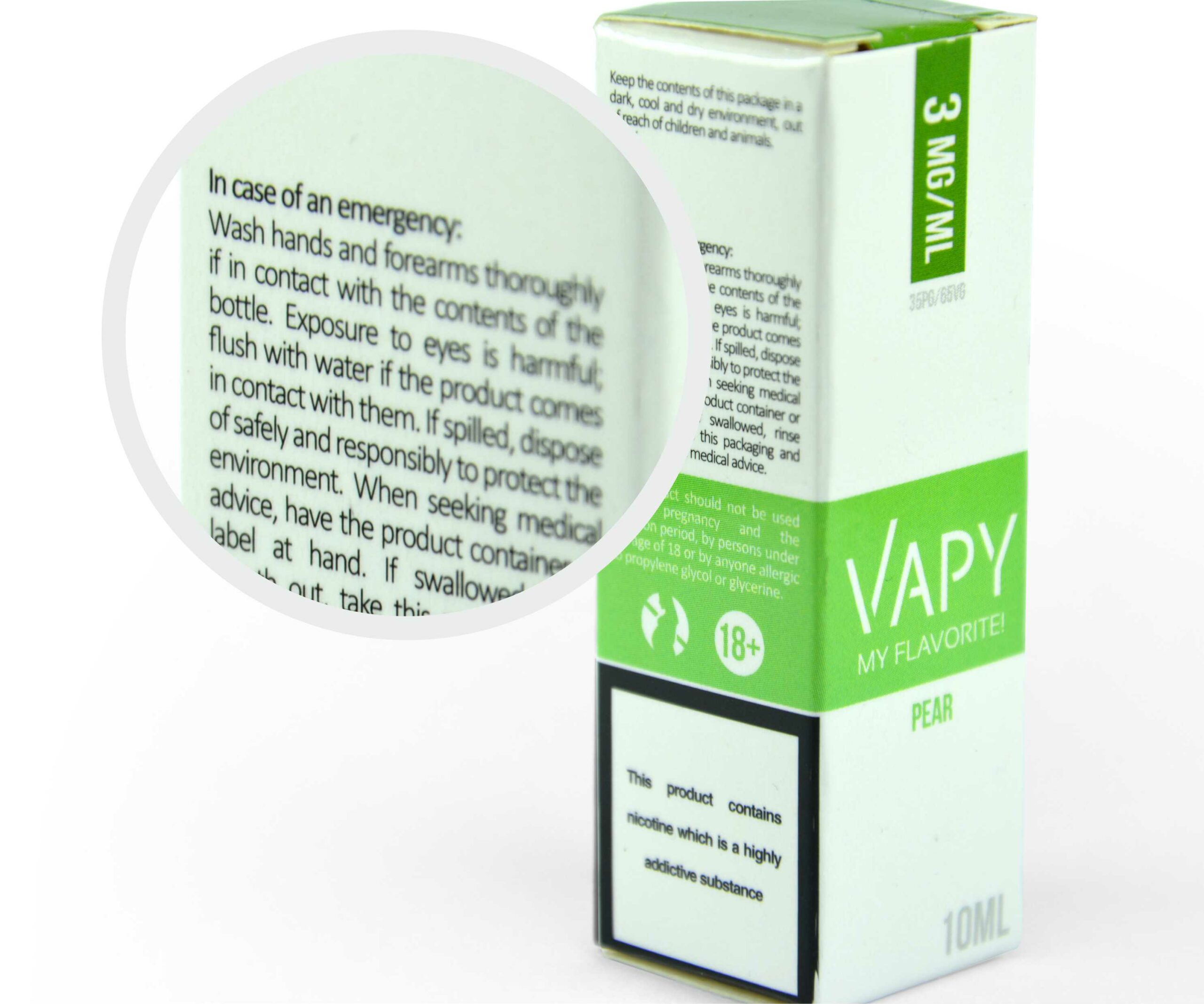 designated for use with e-cigarettes only.” “This product should not be used during pregnancy and the lactation period, by persons under the age of 18 or by anyone allergic to propylene glycol or glycerin.” “Keep the contents of this package in a dark, cool and dry environment, out of reach of children and animals.” “Wash hands and forearms thoroughly if in contact with the contents of the bottle. Exposure to eyes is harmful, flush with water if the product comes in contact with them. If spilled, dispose of it safely and responsibly to protect the environment. When seeking medical advice, have the product container or label at hand. If swallowed, rinse mouth out, take this packaging, and seek immediate medical advice.” “This product contains nicotine which Is a highly addictive substance.”
designated for use with e-cigarettes only.” “This product should not be used during pregnancy and the lactation period, by persons under the age of 18 or by anyone allergic to propylene glycol or glycerin.” “Keep the contents of this package in a dark, cool and dry environment, out of reach of children and animals.” “Wash hands and forearms thoroughly if in contact with the contents of the bottle. Exposure to eyes is harmful, flush with water if the product comes in contact with them. If spilled, dispose of it safely and responsibly to protect the environment. When seeking medical advice, have the product container or label at hand. If swallowed, rinse mouth out, take this packaging, and seek immediate medical advice.” “This product contains nicotine which Is a highly addictive substance.”

Depending on the country the bottle should also be marked with an embossed tactile triangle symbol.
The above information is a good checklist when designing your e-liquid labels and individual e-liquid packaging. If you are new to the market or not sure if you can handle the label and packaging redesign, please contact us, we are an OEM manufacturer for many brands around the globe and will gladly help. Visit this site for more information.
If you want to know more about TPD-compliant e-liquid packaging, check the second part of the article.
Learn more about e-liquid manufacturing:
- OEM Services And Private & White Label: 9 Reasons To Choose Chemnovatic As The Best Business Partner
- How To Start And Grow E-Liquid Brand?
- Pharmaceutical Quality Of E-Liquids: What You Should Know?
- E-Liquid Manufacturing Standards: Tips For Producers And Brand Owners
- E-Liquid Manufacturers: Best Practices
- Packaging, Storing And Transport: Why Are They Important For Product’s Quality?
- Storing And Handling Pure Nicotine And Nicotine Salts
- Safe Usage Of Chemicals [Guide]
- Additives For E-Liquids: What Do You Need To Know About Them?
- E-Liquid Flavors To Make Your Recipes Stand Out
- Natural Vaping Products: Bases, Nicotine, Flavorings
Let’s grow your business together!
Subscribe to our newsletter and receive a free access to our e-mail course on raw materials for e-liquids production (and more!).
No spam, only valuable content we promise to send you.















We are interested in marketing our E-liquids in the EU and the UK. I have read many of the recent documents on the requirements. Obviously there is still much we have to learn. After reading this post I am wondering how much of the information can actually be put on a 10 ml bottle label. do you have any examples of such a label? Can some of this simply be on the exterior container? And what if you don’t have an exterior container for the bottle?
Any advice would be appreciated. We are looking for some council on all the TPD compliance matters.
Eric
Hi Eric,
We have designed the labels for VAPY e-liquids with the TPD in mind, hence they contain all the information required by the Directive.
As for the exterior container , the TPD does not require e-liquids to have a box, the term “individual packaging” may as well refer to the bottle itself, nevertheless it still needs to display all the required information on the label, if the bottle is not big enough then it requires a flyer that is permamently attached to the bottle and contains all information required by law. Do not forget that the individual packaging needs to display a nicotine health warning taking up at least 30% of the packaging.
Hope that helps :)
Please send me a packing sample label with all the required information as wel
Hi, I was wondering what absolutely needs to be visible on the label if a label booklet is used. For example, I assume the nicotine health warning that takes up 30% of the front must be visible, but what about ingredient list, contact information, and other warnings? Could those go inside the label booklet?
Hello, do you have a box and bottle template available so we can put some designs together? thanks
Hi, Mark!
If U want, we can take care of your e-liquid brand – from recipes to graphic design. Let’s make great deal – check our OEM services and contact us rapidly.
Great job! Your article is so informative. I really like it.
Thank you! :) Be sure to subscribe to our newsletter to be up to date with newest information from vaping industry: https://chemnovatic.com/newsletter/
TPD-compliant e-liquid packaging plays a crucial role in ensuring product safety and regulatory adherence. By adhering to the EU Tobacco Products Directive (TPD), manufacturers demonstrate their commitment to protecting consumers, particularly through child-resistant designs, clear labeling, and restricted bottle capacities. These measures help to minimize risks associated with e-liquid consumption and handling while promoting transparency and trust within the vaping industry. Ensuring compliance is not only a legal requirement but also a step towards establishing higher safety standards for all users.
Thank you! We recommend checking more articles about regulatory topics on our blog here! :)