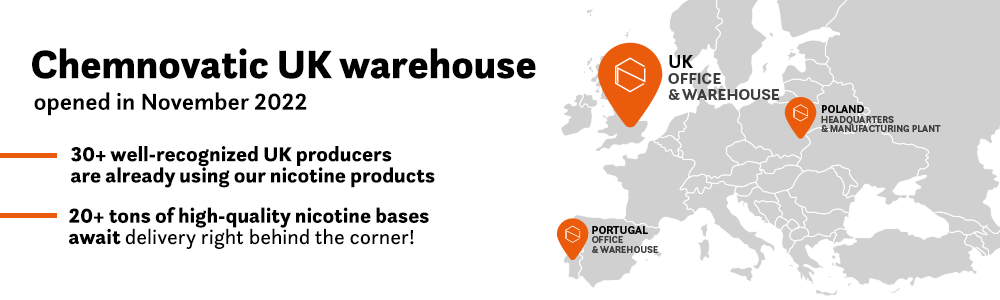
What Brexit means for vaping industry in UK? And will it have any impact on EU TPD notifications? Will Brexit change taxes or export rules? Certainly, many e-liquid producers in the UK or those who are exporting their own products to the UK ask themselves the above questions. The UK left the EU on 31 January 2020. The transition period that was in place – during which nothing changed – ended on 31 December 2020. The rules governing the new relationship between the EU and UK took effect on 1 January 2021. This has resulted in a change of legislation regarding the vape market. In this article, we will try to answer the most pressing questions in a matter-of-fact way.
Learn more:
- UK Vaping Industry: Harm Reduction Plans, Regulations, and Environmental Issues
- E-Liquid Industry Regulations
- E-Liquid Regulations In The World
- E-Liquid Regulations Guide: [Get Your Free E-Book]
- CLP Regulation Changes In 2024: What You Need To Know?
- Tobacco Product Directive (TPD): All You Need To Know
- E-Liquid Industry Regulations: REACH and CLP
- Fees, Taxes And More: An Analysis Of Selected Regulations In Europe
- Excise Duty In The Vape Industry [Guide]
- REACH: What Is Reach And Why We Talk About It?
- REACH and CLP Summary On The Example of PureNic 99+ Pure Nicotine
- TPD Compliant E-Liquid Packaging: Part 1
- TPD Compliant E-Liquid Packaging: Part 2
- E-Liquid Bottles Nozzle Requirements
- What Is UFI Code And How Does It Apply To The E-Liquid Industry?
Change in the UK notification system
There is an important change in terms of TPD regulations, as a result of Brexit. There is a division within the UK into Great Britain (England + Wales + Scotland) and NI (Northern Ireland). For GB territories, a new separate English TPD system is created for reporting new products from the new year, that is, from the end of the so-called transition period after Brexit (i.e. from 1 January 2021).
Products on the Northern Ireland market are to be notified in the same way with the current system, as for the entire EU. The authorities have updated the EU system before the end of 2020 so that it is no longer possible to select the UK as the target market. In return, it is possible to select only the Northern Ireland market.
What is important, notifications existing before Brexit are still valid after Brexit. Such notifications are automatically transferred from the EU system to the new system for GB countries. After the migration is completed, you will be able to update them in the new system, add new brands, add annual reports, etc.
How will Brexit affect notification payments?
New rules concerning the UK notification system are important when submitting new products. From the start of 2021 it is possible to indicate in the new English system which markets the product is intended for (whether only GB or GB + NI) and for easier identification, ensuring that products submitted simultaneously to the GB and NI market have the same EC-ID number for easier verification.
This will determine the registration fee to remain at the same level, which is £ 150 per application. The fee will be charged when the product is entered in the GB market only (£ 150) or as soon as the NI market (£ 150), and also only £ 150 (a type of rebate) when reported to both markets through two separate systems.
MHRA (Medicines & Healthcare products Regulatory Agency) is to verify the notification statuses in both systems in order to correctly identify the product, so as not to double the fee. For example: if we want to register a new product throughout the UK, it will require the submission of one product through two different systems, however, we’ll pay £ 150 for only one product. If we are not fancy for the entire UK (only NI or only GB countries), then, depending on the area, you can apply only through one system, but the fee will also be £ 150.
New Great Britain’ system for e-liquid notifications
The New GB system for e-liquid notifications is a portal that MHRA previously created for the so-called ‘No-deal’ of Brexit. If anyone has set up such an account before, the password and login should still be active and work. Read the step-by-step instructions on how to create an account on this new system. The possibility of reporting new products through the new system is available from January 1, 2021. As for the existing registrations, authorities have migrated current notifications at the end of 2020.
Important information for EU companies wishing to register their products on the UK market! Such companies must establish an entity or person responsible for the GB market, which is inside GB. This will also work the other way around, i.e. companies from GB, when registering on the EU market, must establish an entity / responsible person in the EU (within the EU).
There are two separate public lists, one for GB countries and one for NI. The principle of not waiting 6 months before the notification is effective will still be valid. If the authorities positively approve the product, you can find it on the public list, and from then on you can legally sell it. Other current rules are to remain unchanged (packaging requirements, nicotine-free liquids, annual reports, etc.). New registration will require the same information as before.
Most important changes in vaping industry in UK after Brexit
Important things that need to pay extra attention to are: establishing a representative in GB for EU companies for products registered in GB, and such data will have to be provided at the first possible registration update in the new system for GB. It will be similar for companies from GB on the EU market. And also the toxicological data requirements will become more and more restrictive. This may affect the longer product acceptance time.
Let’s talk!
If you have any questions or comments, feel free to contact us at sales@chemnovatic.com. We are always happy to share our professional knowledge and provide tailor-made advice. Together we can do more!
Let’s grow your business together!
Subscribe to our newsletter and receive a free access to our e-mail course on raw materials for e-liquids production (and more!).
No spam, only valuable content we promise to send you.